Bruton tyrosine kinase inhibitors (BTKi) have generated remarkable clinical responses in patients with chronic lymphocytic leukemia (CLL) and other mature B-cell malignancies. However, deep, and durable response to BTK inhibition is uncommon. A significant number of patients with measurable residual disease relapse with resistance to therapies. Although mutational mechanisms responsible for resistance have been well described, it is not known what mechanism led to persistence of the minimal residual disease (MRD) in the first place.
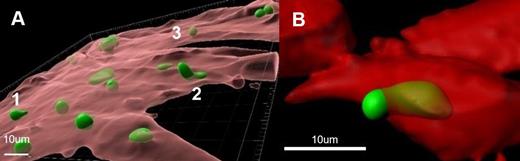
The tumor microenvironment (TME) contributes to drug resistance. Various roles for stroma participation have been described, however, none of these include the physical intracellular sheltering of tumor cells. Cell-in-cell (CIC) describes the microscopic observations of the embodiment of an intact cell by another whole cell ( Fig. 1A). Pathologists have reported this phenomenon sporadically in human fixed tissues for over a century. However, its biological, pathological, and clinical significance remains obscure. In the absence of systematic studies, understanding the significance of live CIC remains challenging.
In this investigation, we studied primary human CLL samples using an ex vivo co-culture model system which mimics the CLL TME. In the TME system, CLL cells were cocultured with a primary bone marrow fibroblast line (BMF) in the presence of B-cell growth factors. The tumor cells survive for weeks, become larger, downregulate surface CXCR4. Phenotypically, tumor cells proliferate and divide into daughter cells. Collectively, these features recapitulate the behavior of lymph node-resident CLL cells. Using the TME system, we have demonstrated that modeled drug response correlates well with patient clinical response to BTK inhibitor ibrutinib and BCL2 inhibitor venetoclax.
Interestingly, with confocal microscopy and 3D reconstruction, we observed that CLL cells placed in the model system were actively internalized by BMF in all 41 CLL cases studied. CLL cells were live and mobile. Moreover, one CLL cell was captured halfway in ( Fig. 1B). The number of internalized CLL cells was quantified and correlated with patients' clinical and pathological features. No correlations were found between CIC and widely used CLL prognostic indicators, including cytogenetic features and IGHV mutational status. However, we have found that patients who were on treatment for CLL at the time of sample collection had higher levels of CIC than those not treated.
We hypothesized that live CIC may be an important mechanism contributing to residual disease persistence and drug resistance. To test the hypothesis, we exposed CLL cells in the TME model system to covalent and noncovalent BTKi including ibrutinib, zanubrutinib, and pirtobrutinib at clinically achievable concentrations. Our results showed that drug exposure drove CLL cells into BMF. This happened specifically to the BTK inhibitors as BCL2 inhibitor venetoclax was not observed to cause such an increase in CIC. These laboratory findings are consistent with the clinical observations that the rate of MRD negativity is much higher with venetoclax treatment than with BTKi.
Additionally, CIC was observed in both follicular lymphoma (FL) cell line and primary FL cells suggesting the phenomenon is not limited to CLL. Further, we found that CIC was stimulated by several B-cell growth factors. The chemokine CXCL12-CXCR4 axis played a critical role in this process. Specifically, conditioned medium containing CXCL12 ligand promoted cell internalization, while plerixafor, a CXCR4 antagonist, largely prevented CIC from occurring. Furthermore, plerixafor blocks ibrutinib-driven CIC in cell lines as well as in primary CLL cells.
Our study represents the first report implicating the CIC phenomenon in residual disease persistence using a cohort of patient-derived CLL cells assessed with 3D imaging and quantitative approach. These findings suggest that live CIC may be an important mechanism tumor cells use to avoid harmful drug insults, survive, persist, and later proliferate, eventually leading to clinical relapse. Targeting the CXCL12-CXCR4 axis may become a potential therapeutic strategy to minimize residual disease and future relapse.
Fig.1. A. Images are reconstructed in 3D using Imaris v10.0. BMF cells are visualized as transparent red surfaces and CLL cells as green surfaces. B. One CLL cell was captured halfway in.
Disclosures
Ma:Genentech: Consultancy; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Eli Lilly and Company/Loxo Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal